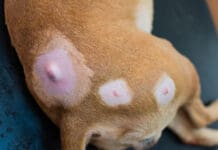
Mast cell tumors (MCTs) are the most common form of malignant skin cancer in dogs, accounting for approximately 7% to 21% of all skin tumors. As there is significant variability in the biological presentation from dog to dog and even from tumor to tumor, this form of canine neoplasia is often referred to as “complicated” and “challenging.”
For more information on the causes of mast cell tumors and how to diagnose them, read our other articles:
Mast Cell Tumors in Dogs: Is It Always Cancer and About Mast Cell Tumors in Dogs.
What are the treatment options for dogs with mast cell tumors?
In the June 2019 issue of Whole Dog Journal, we highlighted a few potential treatment options for dogs with mast cell tumors undergoing research. Two of those treatment options have shown positive progression.
Nanoshell technology and laser ablation for treatment of mast cell tumors
Companion Animal Health continues to explore the use of nanoshell technology and laser ablation for treatment of mast cell tumors. The initial data from one study was presented to the American College of Veterinary Internal Medicine (ACVIM) in 2018 and revealed that 100% of the patients responded to the therapy, with 67% maintaining remission. The treatment combines laser light therapy with gold nanoparticles, which have demonstrated an increased sensitivity to visual and near-infrared light.
The nanoparticles, administered by intravenous injection, congregate in cancerous tissues. The tumor is then irradiated with laser light, causing the electrons within the nanoparticles to enter into an excited state, which releases energy through heat production. This results in an overheating of the regional tissue, with local cell death and destruction following. This non-surgical, one-time treatment option may allow veterinarians to shrink and stop tumor growth in dogs who have masses in regions where surgery may not possible or entirely successful and has limited to no complications.
The FDA recently approved STELFONTA
In November 2020, the U.S. Food and Drug Administration’s Center for Veterinary Medicine (CVM) approved QBiotic’s STELFONTA for the pharmaceutical treatment for all grades of canine non-metastatic mast cell tumors. STELFONTA is a novel veterinary anticancer product containing tigilanol tailgate (also known as EBC‐46), a compound extracted from the seeds of Fontaine picrosperma (commonly known as the brushwood tree) found in the rainforest of North Queensland, Australia. Tigilanol tailgate (TT) triggers the action of enzymes called protein kinase C (PKC); when injected directly into the tumor, it causes a fast but highly localized immune response, disrupting the tumor’s blood supply and thereby inducing tumor cell death. This process leads to the destruction of the tumor mass followed by rapid healing of the resulting wound with minimal scarring.
In a randomized controlled clinical two-phased study involving 123 dogs with cytologically diagnosed MCT, researchers found that a single TT treatment resulted in complete response (tumor completely disappeared) in 75% of cases after 4 weeks (Phase 1). Those dogs who had failed to achieve tumor resolution after 4 weeks were treated with a second dose, and approximately half responded (Phase 2), increasing the overall complete response rate to 87%. Of the treated dogs with complete responses available for follow-up, 100% were still disease-free at the treated tumor site after 8 weeks, and 96% remained disease-free after 12 weeks.
STELFONTA has been approved to treat non-metastatic cutaneous mast cell tumors, and non-metastatic subcutaneous mast cell tumors located at or distal to the elbow or the hock in dogs, and tumor size cannot exceed 10cm. A regimen of corticosteroids and antihistamines/H2 blockers must be given to reduce the risks of severe systemic adverse reactions from mast cell degranulation. Administration of the treatment is by a veterinarian, with a single injection directly into the tumor; a second dose may be given if tumor tissue remains four weeks after the first treatment and the surface of the remaining mass is intact. The most common adverse reactions included wound formation (though this is expected due to the destruction of the tumor), injection site reactions such as mild to moderate pain at the time of injection, reddening/swelling/bruising/thickening of the skin, pain and/or lameness in the treated limb, vomiting, diarrhea, and low albumin levels in the blood. These adverse events were typically low grade, resolved quickly, and usually directly associated with TT’s mode of action. Overall, this innovative treatment has been shown to be well-tolerated, allowing dogs to regain quality of life quickly.
STELFONTA will be launched in the United States by global animal health company Virbac, with availability to veterinary oncologists in the next few months; availability to primary care veterinarians will follow.




I lost my best dog to Hemangiosarcoma in 2017. While that’s different than mast cell tumors, I was shocked when I learned just how prevalent cancer is in our best friends. I have learned so much on my journey and doing everything in my power to prevent my two beloved dogs from developing cancer. I am happy to learn of more advanced treatment options for our best friends.
We just lost our 9 year old rescue dog to cancer. It pains me to think of my husband hollering at the dogs for barking or just being dogs, terrifying them. (He is mentally ill due to the abuse he suffered as a child. He has a hard time controlling his anger.) If only we were kind to our dogs before they became terminal. A lesson for all.
We just lost our dog to spleen cancer .. he was a pit pull lab mix and he was the sweetest dog ever . He was 13 years old . We miss him sooooo much . He was put down and it’s so hard right now 😢